Can You Eat Metal?
Approximate time to complete: 25-30 minutes + questions and journal time

Approximate time to complete: 25-30 minutes + questions and journal time

This experiment is a wonderful extension to combine some of the things you learned from the following topics.
Is there really metal in your food? Can you extract it? (Can you get the metal out?)
First thing first: DO NOT TRY EATING METALS. Many metals are not safe to eat, and even the ones that are can be toxic in high doses.
Note: 300mL = Approximately 1 ¼ Cups






If you have younger ones, save the bag of cereal solution for a while longer! The cereal will soak up even more water and make for an interesting sensorial experience.
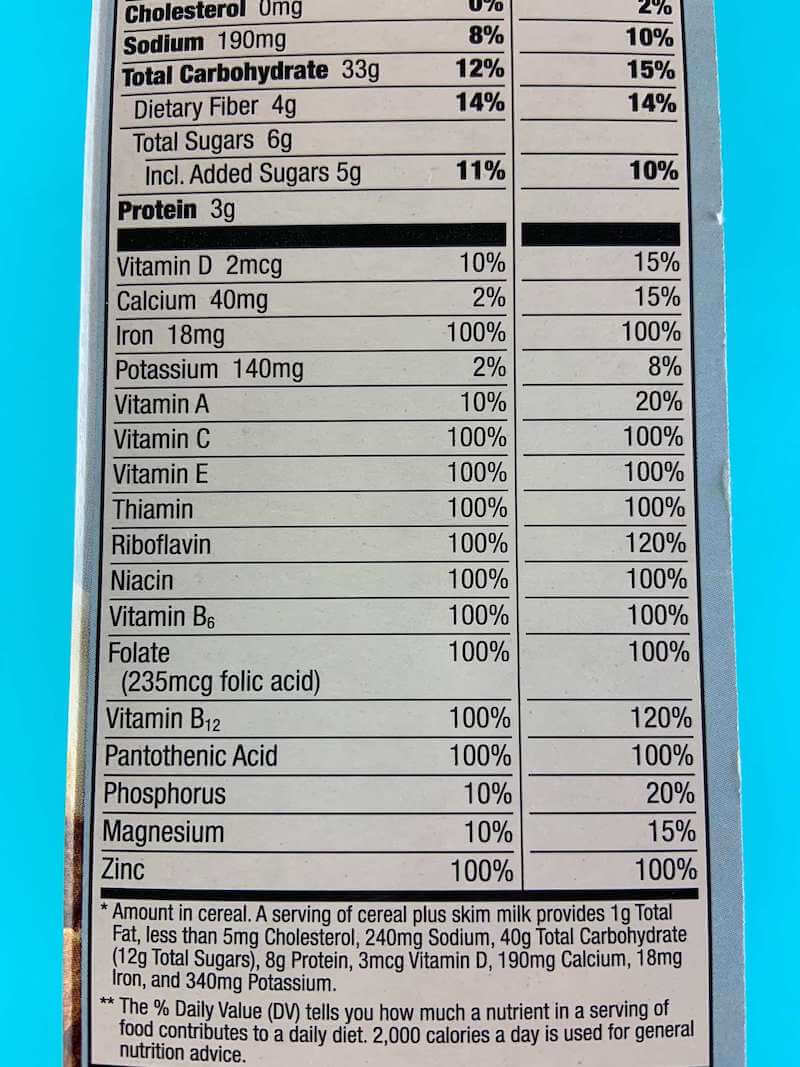
There are metal elements that are found in our bodies, and in our foods. Some examples are magnesium, zinc, and iron. These metals are minerals that our bodies need to function, but that we cannot produce ourselves. They are called essential minerals, and we need to eat them to get them. If you look at the nutrition label on your food, you can see what is in it. Some foods, like certain breakfast cereals, have A LOT of iron in them. The one we used for this experiment has 100% of your daily value of iron.

When you crush up the breakfast cereal and add it to water, part of the cereal dissolves in the water to make a solution. The iron, however, does not dissolve and floats freely through the water.
We know from experimenting with iron filings and magnets in Combining and Separating that iron will “stick” to magnets. In other words, iron is attracted to magnets.
When you pass a magnet over the cereal solution, the magnet attracts the free-floating iron, and you can see the little pieces of iron that are in your breakfast cereal.

Are you interested in seeing what Montessori Laboratory’s big-picture lessons, hands-on experiments, and engaging science activities are all about? Check out the free lessons below!
Want more lessons like these ones? Get access to all of our lessons with a Montessori Laboratory membership!