Crystallization: The Art of Nature’s Tiny Wonders

Introduction: A World of Crystals
Are you excited to learn about crystallization, the process that turns simple things like salt and sugar into beautiful crystals? Think about the beauty of a snowflake or a sparkling gemstone – these are perfect examples of crystallization that everyone can appreciate.
In this article, we’ll explore the amazing world of crystallization. We’ll see how it creates everything from the unique designs of snowflakes to the natural crystals found in rocks. Let’s start our journey into the tiny, fascinating world of crystals and learn about their importance and beauty.
Key Takeaways
- What is Crystallization?: Salt and sugar are made of tiny crystals. The process that creates these crystals is called crystallization. It’s a fascinating journey that involves four steps: dissolving substances in water, creating the right mix, starting a tiny crystal, and then watching it grow!
- What Shapes a Crystal?: Have you ever wondered why different crystals look different? The shape and size of a crystal depend on things like how warm or cool it is and how much of the substance is in the water. These factors are the secret recipe for each unique crystal we see.
- All Kinds of Crystals: Crystals come in all sorts of shapes, sizes, colors, and textures. It’s amazing how many types there are, and each one forms under its own special conditions.
- Crystals All Around Us: Crystals aren’t just pretty; they are used in things like electronics, art, jewelry, and even in nature. They are everywhere, showing us how important they are in our lives.
- Learning with Crystals: The Montessori Laboratory membership includes a big-picture lesson on crystallization. It’s perfect for young minds who love hands-on experiments and seeing things for themselves.
- Nature’s Crystal Art: Nature is an amazing artist, especially when it comes to making things like snowflakes, geodes, and stalactites. These are all beautiful examples of crystallization in the natural world.
- Why Crystals Matter in Science: Scientists are always discovering more about crystals. This is really important for making new medicines, creating materials, and helping the environment. Crystals are not just cool to look at; they help us in so many ways!

Simplifying Crystallization: How Nature Crafts Its Treasures
Crystallization may seem complex, but it’s really a natural and beautiful process. It’s about how substances like salt and sugar, which are already crystals on a tiny scale, transform to form larger, well-structured crystals. This transformation is a great example of a phase change.
The Ingredients of Crystallization
Every crystal begins with basic components. The main one is the crystalline substance itself, like salt or sugar. These substances are already crystals in a microscopic form. The other key component is a solvent, like water, that dissolves the substance. When these ingredients come together under the right conditions, they start the process of forming larger, well-structured crystals.

The Phase Change in Crystallization
A phase change in crystallization refers to the transition from a dissolved state in a liquid (like salt in water) to a solid state (the crystal). This change is fascinating because it involves rearranging molecules into a more ordered, structured form.
The Steps of Crystal Formation
- Solution Formation: The substance (salt or sugar) is dissolved in the solvent (water). This is the start of the phase change, as the solid substance becomes part of a liquid solution.
- Saturation: The solution reaches a point where no more substance can dissolve. This balance is crucial for the next steps.
- Nucleation: The initial formation of tiny, solid clusters from the dissolved substance marks the beginning of the return to a solid state. These clusters are the “seeds” of the larger crystals.
- Crystal Growth: The final phase where the small seeds grow as more of the dissolved substance joins them, transitioning back to a solid state and forming larger, visible crystals.

Understanding the Process
Each step in this process is important for forming well-structured crystals. If conditions like temperature or concentration vary, it can affect the crystal’s appearance. Under ideal conditions, however, the result is a larger, visible crystal that has transformed from the tiny crystals we started with.
In the next section, we’ll explore how temperature and concentration are key players in this crystal-making adventure.
The Role of Temperature and Concentration in Crystal Creation
To fully understand crystallization, we need to explore the roles of temperature and concentration. These two factors greatly influence how and when crystals form.
Temperature: The Crystal’s Environment
Temperature is a key factor in crystallization. In environments where a hot solution can form and then cool, crystals begin to form. An example of this kind of environment is a geothermal area. Crystals also form in places where water repeatedly evaporates – like a salt flat.
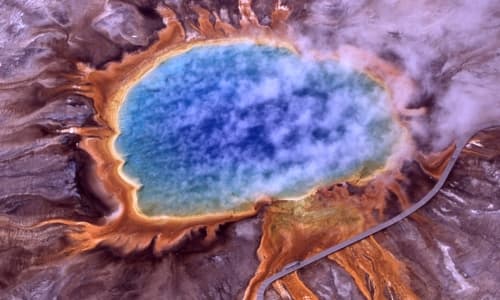

The warmer the environment, the more substance can dissolve, leading to a richer solution. As the environment cools, the dissolved substance starts to form crystals. However, there is a limit; extremely high temperatures can disrupt the crystal formation process, preventing crystals from forming properly.
Concentration: The Crystal’s Building Blocks
Concentration, in contrast to temperature, is about the amount of substance dissolved in the solvent. A solution reaches saturation when it is at room temperature and cannot dissolve any more of the substance. This saturation point is critical for crystal growth. You can add more substance to a saturated solution by raising the temperature, at which point it becomes a supersaturated solution. Supersaturated solutions crystallize more easily.
Crystallization Experiments with Montessori Laboratory
With your Montessori Laboratory membership, you have access to hands-on experiments that allow young learners to see the principles of crystallization in action. For instance, creating crystal ornaments with Epsom salt can illustrate how crystals form different shapes. Experimenting with both cold and hot water solutions shows how temperature affects crystal formation. These activities demonstrate temperature’s role in crystallization and help children see the impact of these factors on crystal growth.
These practical experiences turn abstract scientific concepts into visible and exciting realities. By understanding the roles of temperature and concentration, children gain insight into the delicate balance nature maintains to create the diverse and beautiful world of crystals around us.

Exploring Different Types of Crystals
The world of crystals showcases nature’s incredible diversity, each type distinguished by its unique shape, size, and color. We’ll explore the fascinating variety that exists in our world.
The Many Shapes and Sizes of Crystals
Crystals form in various geometric patterns that each have a specific look:
- Cubic Crystals: As the name suggests, these crystals are cube-shaped. One common example is table salt. Pour some out and look at it under a magnifying glass to see!
- Hexagonal Crystals: These are six-sided prisms that are long and elegant. Quartz is a well-known example.
- Tetragonal Crystals: These are similar to cubes, but stretched along one axis to create an elongated structure. An example is wulfenite, which is known for its fascinating shapes.
- Orthorhombic Crystals: These crystals have a box-like shape where all sides have different lengths. Topaz is a mineral that often forms in this pattern.
- Monoclinic Crystals: These crystals have slanted, asymmetrical structures. Gypsum, which is used in drywall, is a common monoclinic crystal.
- Triclinic Crystals: Known for their intriguingly irregular shapes, these crystals have no right angles. Turquoise is an example, with its unique and captivating form.

Looking at all these different crystals shows us the many ways that nature mixes things up to make so many different crystal shapes.
Colors and Textures
Crystals also vary widely in color and texture:
- Colorful Varieties: Some crystals, like amethyst, get their color from trace minerals or impurities. Others, like sapphires, get their color from the specific arrangement of atoms.
- Textures and Clarity: Crystals can range from the smooth, glassy surface of obsidian to the rough, granular feel of sandstone. There are so many different textures and clarity levels.

Crystal Formation: A Closer Look
Understanding crystal formation unravels one of the mysteries of nature. Factors like temperature, pressure, and chemical composition determine a crystal’s final shape and size. You can see this process happening in everyday life, from frost forming on a window to sugar crystallizing in a supersaturated solution during candy-making.
Crystals in the Classroom and Home
For children, studying crystals is a fascinating way to explore science and appreciate nature’s artistry. Simple activities, such as growing salt crystals or examining sugar crystals under a magnifying glass, offer fun and educational experiences.


Crystals in Everyday Life
Have you ever spotted crystals around your home or classroom? You might be surprised to find that they are everywhere in our daily lives!
In Technology
Did you know that crystals help us tell time? Quartz crystals in watches and clocks tick away, keeping the time just right. And that’s not all – silicon and other crystals are super important in making gadgets like your smartphone and computer work!
In Jewelry and Art
Crystals are also stars in the world of jewelry and art. Think about sparkling gemstones like diamonds, rubies, and emeralds. These aren’t just pretty to look at; they have special properties that make them fascinating to learn about. They’re like tiny, natural treasures that artists and jewelers love to use.

In the Natural World
There are more crystals in nature than just snowflakes and salt. Have you ever seen pictures of giant crystal caves or the beautiful crystalline structures of geodes? These are all natural crystals formed over thousands of years. Even the chalk used in classrooms is made from tiny crystals formed from ancient sea creatures!

Inspiring Curiosity
All these examples of crystals around us are perfect for sparking curiosity. Next time you look at a watch or a geode, think about the cool science behind them. These little wonders can lead to big questions and discoveries about science and nature.
Crystals make our world more interesting in so many ways. They’re not just for scientists to study; they’re a part of our everyday lives. They give us lots of chances to learn, explore, and be amazed.
Fun Crystal Experiments: Learning by Doing
Doing hands-on crystal experiments is an amazing way for children to dive into the world of crystallization. These activities are not just fun; they bring scientific concepts to life and spark curiosity and imagination.
A World of Discovery at Your Fingertips
At Montessori Laboratory, we encourage exploration through engaging experiments like these:
- Examining Salt and Sugar Crystals: In this experiment, children dissolve salt and sugar in water and observe their crystallization on Petri dishes. Under a magnifying glass, they can see the detailed and unique crystal structures of these everyday ingredients.
- Comparing Crystals: By placing small amounts of salt, sugar, and Epsom salt on separate Petri dishes, children get to compare the different shapes and colors of these crystals. This activity showcases the variety in crystal structures, even among familiar substances.
- Growing Crystals at Home: Using Epsom salt, hot water, and a pipe cleaner, children can grow their own crystals. This hands-on activity helps them understand how crystals form around objects by demonstrating nucleation and crystal growth in a practical and exciting way.

These experiments provide valuable insights into crystallization, which makes this complex process understandable and intriguing for young learners.
Inspiring Young Minds Through Science
For educators and caregivers, these experiments are opportunities to ignite a love for science in children. Each activity is a lesson in observation and critical thinking. For example, when children grow crystals at home, they’re not just watching a pretty structure form; they’re learning about how changes in temperature and concentration affect crystal growth. They might wonder why some crystals grow larger or faster than others, leading to discussions about the science behind crystallization.
These experiments go beyond the classroom – they can inspire children to notice and appreciate the intricate patterns in nature, from the frost on a leaf to the crystalline structure of a seashell. By engaging with these scientific phenomena hands-on, children develop a deeper appreciation for the natural world. These activities are gateways to discovery, encouraging young minds to explore, question, and understand the world around them.

Crystallization in Nature: More Than Just Science
Crystallization isn’t just something that happens in a lab; it’s a part of nature’s art, creating beautiful wonders that are both fun to look at and learn about.
The Artistry of Ice and Snow
Think about snowflakes. Each one is special, with its own crystalline pattern. As snowflakes freeze, they create hexagonal shapes, which is a neat example of crystals forming in the sky!

Geological Wonders
Under the ground, there are rocks called geodes. They might look ordinary on the outside, but inside, they are full of amazing crystal formations. When you split open a geode, it’s like finding a hidden treasure made by nature.

Caverns of Crystal
Deep in limestone caves, where water drips slowly, crystals form over many, many years. Stalactites hang down from the ceiling like icicles, and stalagmites rise from the ground. Together, they look like a forest made of crystal columns. This shows how crystallization can take a long time but creates something really amazing.

Crystallization in Everyday Life
While we’ve already talked about the crystals in sugar and salt, there’s more to discover right around us. In your garden, you might notice tiny crystals forming on leaves after watering – a mini spectacle of nature’s crystallization. Or when you’re outside, have a look at the pebbles and rocks. Many of them glitter because of small crystals inside, like quartz or mica. These everyday items are tiny windows into the fascinating world of crystals.

The Continuing Journey: Discoveries in Crystallization
Continuing on our exploration of crystals, let’s look at some amazing recent discoveries having to do with crystallization:
- Unraveling Protein Crystals: Scientists are figuring out the complex structures of protein crystals. These crystals help us know how proteins work inside our bodies. Proteins are like tiny machines in our cells that do important jobs. Understanding them can help us make new medicines and learn more about how our bodies work.
- Advancements in Materials Science: Thanks to crystallization, we’re finding new ways to make materials that are better and more efficient.
These discoveries aren’t just for scientists in labs; they matter in our everyday lives too. For example, understanding crystals helps make new medicines in the pharmaceutical industry. It’s also important in environmental science, where knowing about crystals can help with things like pollution control and managing our natural resources.
Remember, every crystal has a story. From tiny snowflakes to huge geodes, they all tell us about the wonders of nature and the efforts of scientists to understand our world.

Responses