How Did Humans Discover Fire?
-
Prep
Planning & Materials -
Discussion
-
Video LessonsHow Did Humans Discover Fire?
-
Experiments and ActivitiesThe Fire Triangle
-
How to Put a Fire Out
-
Can Metal Burn?
-
Make Your Own Fire Extinguisher
-
Jumping Flames
-
Fire Projects & Further Research
-
AnswersAnswer Key: How Did Humans Discover Fire?
Answer Key: How Did Humans Discover Fire?
We encourage you to experiment or do research to find the answers to these questions on your own before looking at our answers!
Discussion
Fire is what happens during a special kind of chemical reaction called combustion. It is bright and hot.
You need fuel, heat, and oxygen to make a fire. These three things are called the “fire triangle,” which you will learn more about in this lesson.
Humans use fire to cook, stay warm, for light, and for other things.
We don’t know for sure how humans discovered fire, but in this lesson, we will tell a story based in science about how it may have happened.
How to Put a Fire Out
Part 3: The Match You Put In Water
The match you put in water took less time to go out than the regular match.
By putting it in water, you took heat and oxygen away from the fire triangle.
Part 4: The Match You Cover
The covered match took less time to go out than the regular match.
You took away the oxygen by covering the match. As the match burns, it uses oxygen, once it uses all of the oxygen inside of the cup, it goes out.
If you use a larger container, the match will go out more slowly. If you use a smaller container, the match will go out more quickly. See the answer key for Can You Put a Fire Out Slowly? for a more in-depth explanation.
Part 5: The Match You Break In Half
The half match went out more quickly than the regular match.
By breaking the match in half, you took away the fuel. The half match burned out more quickly because there was not as much fuel in the form of wood.
It would probably take more time to go out if everything else was equal.
Assuming everything else was equal, a thinner match would probably take less time to go out than a thicker match.
Part 6: Optional Extension
If all of the matches were made perfectly the same, they would take the same amount of time to burn. Look closely at a handful of matches. They are usually not made perfectly the same. Some have a bigger phosphorus cap, some are thinner, some are thicker … any of those differences could cause the matches to burn at different rates.
Some of your matches may burn out before they reach the bottom of the match stick. When you hold a match upward, the flame is traveling up, and away from the wood. The flame is where the heat is and heat rises, so sometimes, there is not enough heat traveling down to light the unburnt wood at the bottom of the match. Without heat (one side of the fire triangle), there is no fire. If you were able to secure a match horizontally so that it could burn all the way to the bottom of the match stick safely, it would burn all the way down every time.
Questions
If you take away any one part of the fire triangle, the fire will go out.
Smothering the fire with water put the flame out the quickest. However, you would not want to use water to put out a grease fire, because water and grease do not mix. Instead, the water will create an explosion as the water quickly heats up from the fire. The water will turn into vapor and send little droplets of flaming grease everywhere. You can put out a grease fire with baking soda, salt if it is small enough to manage.
To put out wildfires, they have to use a combination of different tactics including smothering the current fire with water and removing fuel in its path by spraying it with fire-retardant. Since wildfires are so big, there are many different variables at play.
This experiment was not an equal race. When you dip a flame in water, you immediately remove heat and oxygen. When you cover a flame with a cup, there is still some oxygen left in the cup that can keep the flame alive for a bit. When you remove half of the match, you still have the other half as fuel that the flame can continue to burn through.
Some of these tactics can be used if you need to put a fire out. For example, when camping it is important to make sure that you stop fire from spreading by putting it fully out before you leave. We hope you never need to put a fire out that was started unintentionally, but firefighters do all of the time. They use the same concepts that you used in this experiment for fire management.
*We are not suggesting that you try to start a fire for the sake of extinguishing it.
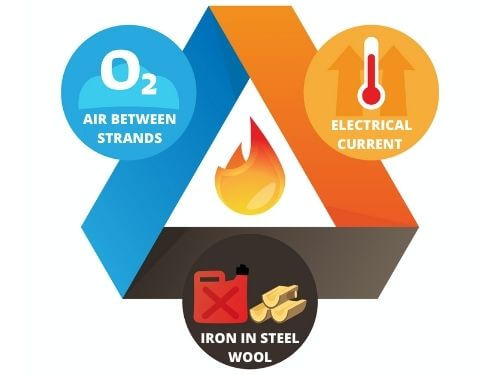
Can Metal Burn?
- Fuel: Steel Wool
- Heat: Electricity
- Oxygen: Air
Yes, metal can burn. Other things that are quick to burn are wood, coal, gas, alcohol, and fats (grease, oil). Almost everything can burn with enough heat.
One group of things that will not “burn” or otherwise be part of an exothermic (heat releasing) reaction are the Noble Gases. Helium is a Noble Gas and is used to fill blimps because it is less dense than air, and non-flammable.
To learn which gases are considered Noble Gases, take a look at your Parts of the Periodic Table Cards.
1292°F (700°C). That is very hot!
In order to get a steel rod to burn, the temperature would have to be very hot. Steel wool burns more easily than steel rods because steel wool has a lot of oxygen between the strands of metal.
When you rub the battery across the steel wool, it creates an electrical current, which generates heat and a spark. Lightning also occurs when particles rub against each other up in the sky, which creates an electrical current. Lightning is the discharge of that electrical current, and it generates heat and a spark just like the battery and steel wool.
Electrical companies prevent electrical fires by surrounding electrical wiring with a non-conductor, something that does not let electricity flow out. They also take care to make sure electrical wiring is not placed near easily combustible material (material that catches fire easily).
Example:
Using steel wool and batteries to make a campfire in the wilderness (just don’t keep them near each other in your pack!) or in your home in case of an emergency like a utility outage.
*We are not suggesting you try to start a fire with steel wool and batteries other than within the confines of this experiment.
The battery creates an electrical current through the steel wool. That electrical current lets off heat. The heat from the electrical current, the oxygen between the steel wool strands, and the iron in steel wool are each one side of the fire triangle. Altogether, they can ignite and hold a flame.

Make Your Own Fire Extinguisher
Part 1
Nothing should have happened when you poured an empty cup (more accurately, a cup full of air) over the flame.
A control group is something in an experiment or study that is not being tested on. It is used as a benchmark to compare the things being tested. In this experiment, we are testing whether or not we can use the gas that the baking soda and vinegar reaction makes to put out a flame. In order to make sure that it is not simply the tipping of the cup that puts the flame out, we tested with our control: an “empty” cup (more accurately, a cup full of air).
Part 2
The flame should have gone out when you poured the cup full of gas (the product of the baking soda and vinegar reaction) over the flame.
Yes, you removed oxygen by smothering the flame with another, heavier gas: carbon dioxide.
Questions
No, the candle should have stopped burning when you poured the carbon dioxide over it.
Yes, fire extinguishers are filled with highly pressurized, non-flammable carbon dioxide.
When you cover a candle with a cup, it has a finite amount of oxygen to burn. As the candle burns, it removes oxygen from the cup and adds carbon dioxide to the cup. Both the lack of oxygen and the smothering of the flame by carbon dioxide stop the flame from burning.
Carbon dioxide is heavier (more accurately, more dense) than oxygen. That is why you are able to keep it in the cup. It is also why you are able to pour it out of the cup, and why it sinks down to the surface where the candle is.
It does, and the different layers are formed due to the density of the gases in each layer. Check out Earth’s Blanket to learn more about the atmosphere and its layers.
Fire needs oxygen to survive. Baking soda and vinegar make carbon dioxide, which you trapped in the balloon. Carbon dioxide is heavier than air, so it sinks down low into the cup, and pushes the oxygen away. When you pour it out of the cup, and onto the flame, it does the same thing. The carbon dioxide sinks down towards the flame, and pushes the oxygen away from the flame. Without oxygen, the fire stops burning.




Jumping Flames
You should not have been able to light the candle without touching the flame to the wick.
You should have been able to light the candle by touching it to the wick.
You should have seen smoke coming from the candlewick.
You should have been able to light the candle without touching the flame to the wick.
Questions
The wax gas is burning. Read the “What’s Happening?” section above for an explanation.
The candle we used is made of paraffin wax. This demonstration should work with any type of candle because all candles create smoke.
You’ll have to test this one out for yourself! The greater the concentration and reach of the smoke, the further the flame will be able to jump. A larger wick is likely to produce smoke with greater concentration and reach.
Wildfires have been known to “jump” over freeways and other non-flammable material.
- Solid wax melts into liquid wax
- Candle wick absorbs liquid wax
- Liquid wax vaporizes into wax gas
- Wax gas + oxygen + heat = fire

Wax doesn’t burn well as a solid. First, it has to melt into a liquid – that’s why you have to start the candle with another flame like a lighter. The candle wick absorbs the liquid wax, and brings it closer to the heat source: the flame. Then, the wax gets so hot that it turns into wax gas. The wax gas interacts with the oxygen in the air and the heat from the flame. The wax gas burns, and turns into carbon dioxide and water vapor.
When you blow the candle out, there is some wax gas that hasn’t turned into carbon dioxide gas and water vapor yet. That wax gas can catch on fire if you put a flame near it, and the flame will travel back down to the fuel source: the candle.